Recently, our center achieved the effective regulation of the reaction pathway of zinc-iron double oxide Fenton-like catalyst through visible light irradiation, providing a new strategy for the modulation of mechanism from radical to non-radical of heterogeneous catalyst in Fenton-like reaction.
The quenching of radical groups in Fenton-like reactions by various inorganic anions or high concentration of organic matter limits their value in industrial applications. Conversely, non-radical-dominated systems can effectively overcome the above limitations, showing high activity against the degradation of contaminants under widespread matrix interference in water. The development of inexpensive, environmentally friendly non-radical-dominated catalysts is one of the priorities of current research.
Here, we have successfully prepared a series of Zn-Fe double oxides by calcining the Zn1-xFex-Fe Prussian blue analogues in an ambient atmosphere. Through Mössbauer spectroscopy and combined with various other conventional techniques, it is revealed that the samples ZFO-1 and 4 were composed of nanocomposited ZnFe2O4 and ZnO, while ZFO-2 and 3 consisted of ZnFe2O4 and Fe2O3 (Figure 1).

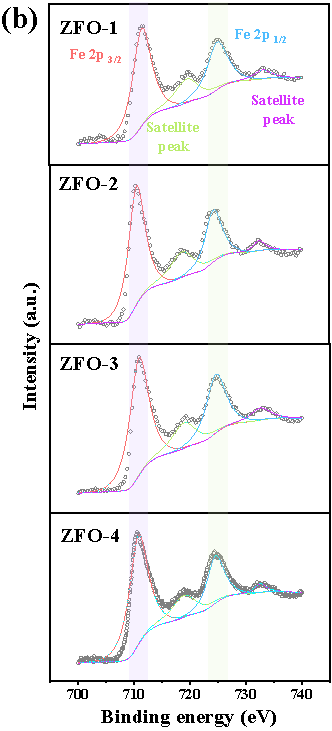
Fig. 1. Room temperature 57Fe Mössbauer spectra (a) and high resolution XPS spectra of Fe 2p (b) of prepared Zn-Fe double oxides.
Under the conditions of visible light irradiation and peroxonosulfate (PMS) co-existing, Zn-Fe double oxides showed excellent catalytic performance for the oxidation of various organic pollutants with the leaching of trace iron, and showed superior pollutant decomposition efficiency in high concentration organic matter humic acid, varous kinds of anions and simulated actual water system, revealing that visible light plays an important role in the PMS-based Fenton-like reaction over Zn-Fe double oxides (Figure 2).
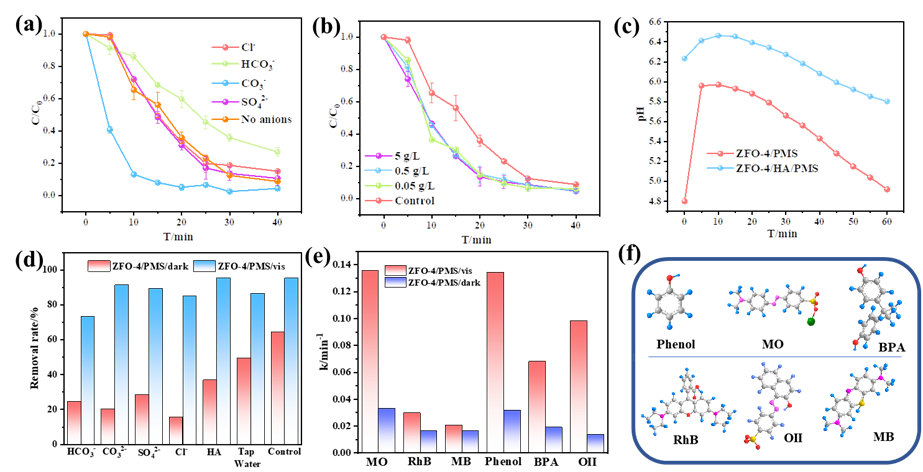
Fig.2.Effect of different anions (a) and humic acid (HA) concentration (b) on the catalytic efficiency of ZFO-4/PMS/vis system; The change of pH under ZFO-4/PMS/vis/HA and ZFO-4/PMS/HA systems (c); The BPA removal rates of ZFO-4/PMS/dark and ZFO-4/PMS/vis systems existing different anions, HA and water quality (d); The constant rate k of different organic contaminants in the ZFO-4/PMS/dark and ZFO-4/PMS/vis systems (e); The structural formula and classification of different organic pollutants (f).
By the radical quenching experiments and EPR spectroscopy characterization, photoinduced electrons and holes can be employed to trigger the efficient activation of PMS, thus transforming the reaction pathway in the prepared Zn-Fe dioxides from the radical pathway to the non-radical pathway dominated by singlet oxygen (1O2). This work provided a new perspective for using solar energy to regulate radical and non-radical pathways in advanced PMS based oxidation processes (Figure 3).
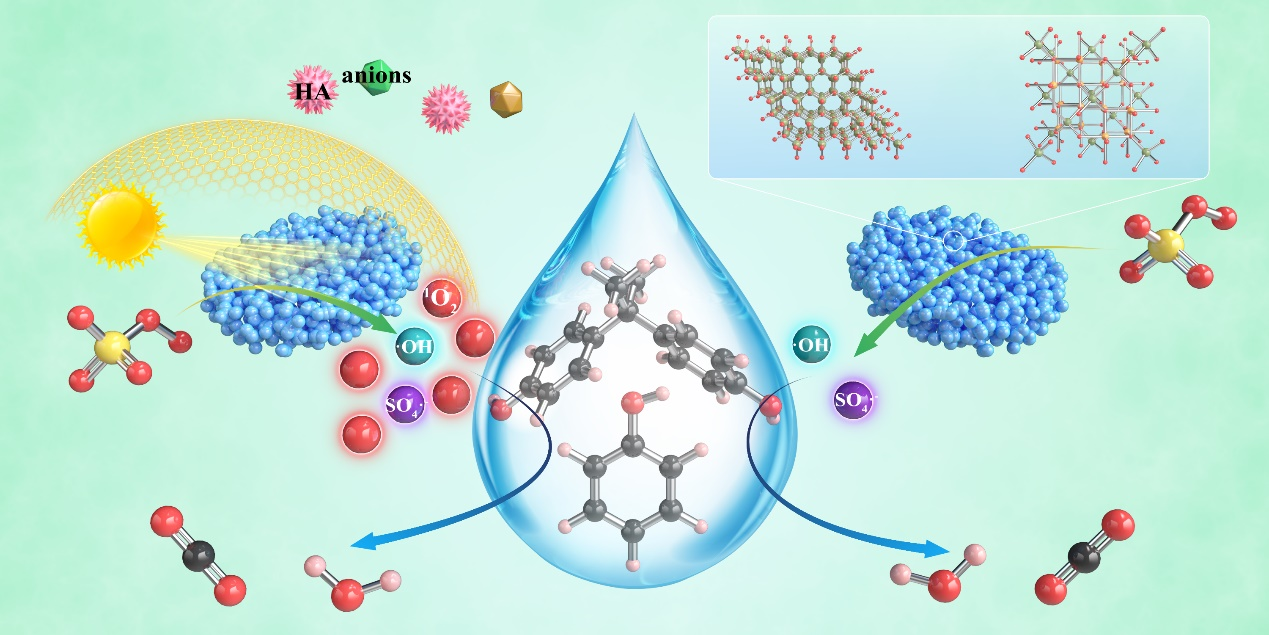
Fig.3. Possible mechanisms of organic pollutant degradation in ZFO-4/PMS/vis system (left) and ZFO-4/PMS system.
The relevant research results entitled "Modulation of reaction pathway of Prussian blue analogues derived Zn-Fe double oxides towards organic pollutants oxidation" has been published in Chemical Engineering Journal, and the research project has been funded by the National Natural Science Foundation of China (21961142006) and International Partnership Program of Chinese Academy of Sciences (121421KYSB20170020).
DOI: 10.1016/j.cej.2022.140103
Relevant link: https://www.sciencedirect.com/science/article/pii/S1385894722055838.